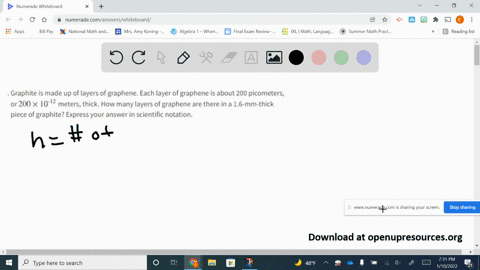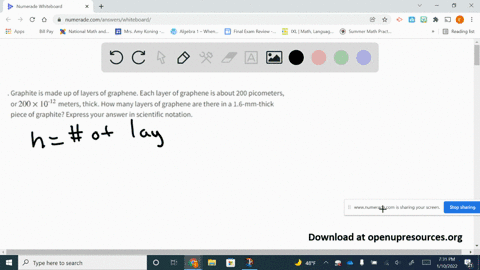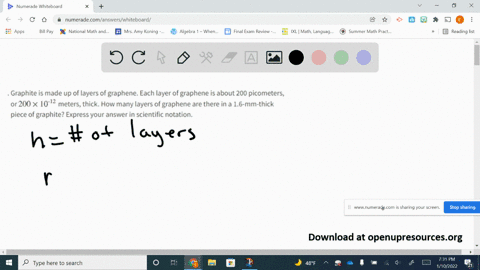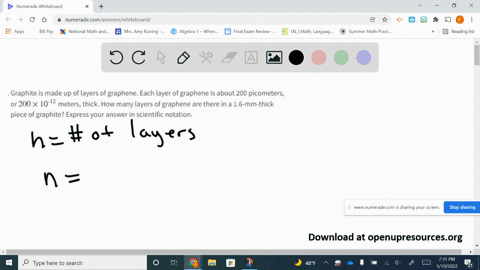
SOLVED:Graphite is made up of layers of graphene. Each layer of graphene is about 200 picometers, or 200 ×10^-12 meters, thick. How many layers of graphene are there in a 1.6-mm-thick piece
.png)
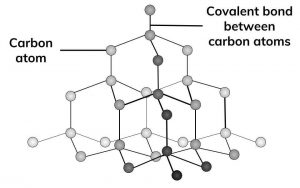
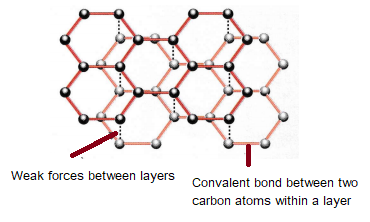
a What is graphite? Of what substance is graphite made? b Describe the structure of graphite with the help of a labelled diagram. c Why is graphite a good conductor of electricity

a) What is graphite? Of what substacne is graphite made? (b) Describe the structure of graphite - YouTube

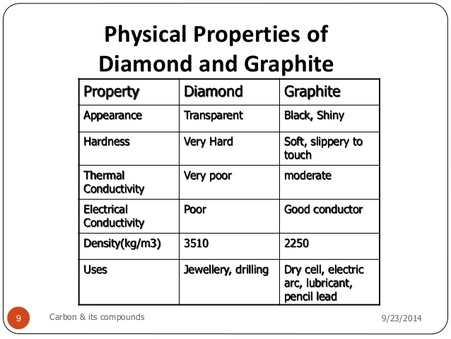
Graphite Graphite is made up of carbon atoms. Chemical formula: C It is an allotrope of carbon. Ex. Graphite vs. Diamond Graphite has the same. - ppt download

a) What is graphite? Of what substance is graphite made?(b) Describe the structure of graphite with the help of a labelled diagram.(c) Why is graphite a good conductor of electricity but diamond

Solved: The diagram above shows the crystal structure of graphite, a mineral made up of carbon atoms that - Brainly.com

TIL There is no lead in "lead pencils". Rather, the core is made up of a non-toxic mineral called graphite. The common name “pencil lead” is due to a historic association with
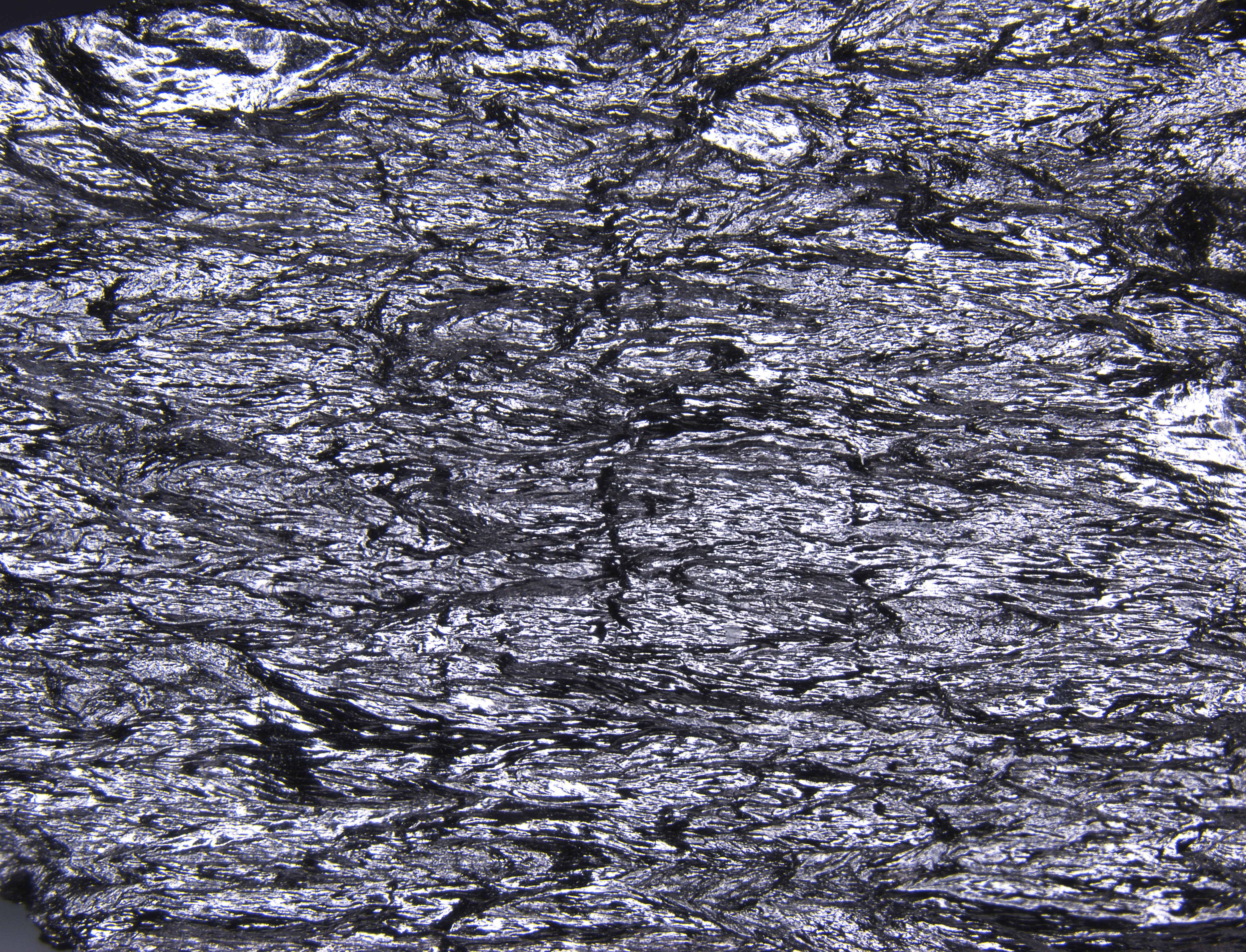
How can graphite and diamond be so different if they are both composed of pure carbon? - Scientific American

a What is graphite Of what substance is graphite made b Describe the structure of graphite with the ...